Pitolisant was approved by the U.S. Food and Drug Administration (FDA) in August 2019.[5] It was granted orphan drug designation for the treatment of narcolepsy, fast track designation for the treatment of excessive daytime sleepiness (EDS) and cataplexy in people with narcolepsy, and breakthrough therapy designation for the treatment of cataplexy in people with narcolepsy. Pitolisant represents the first new therapy in the U.S. in over fifteen years for the treatment of both EDS and cataplexy in adults with narcolepsy.
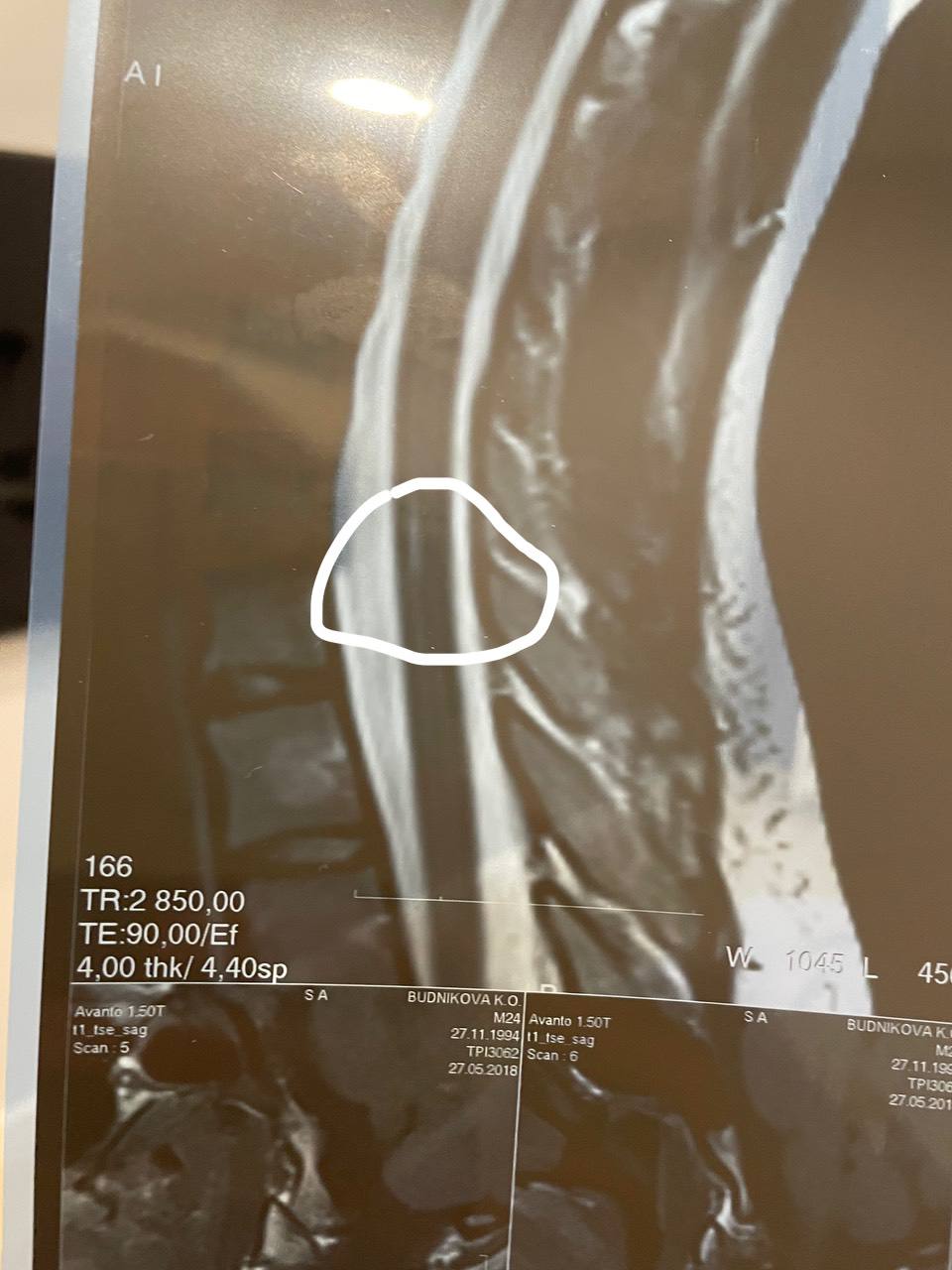
Интересно, есть ли шансы что его будут завозить?
https://en.wikipedia.org/wiki/Pitolisant